You have chosen not to allow videos from the 3rd party streaming service (YouTube), if you would like to see these videos, please change your Privacy policy and cookie settings.
State of the fertility sector 2021/22
Annual publication on HFEA licensed clinics
Published: October 2022
Download the underlying dataset as .xlsx
Table of contents
- About this report
- 1. Changes to interim inspection themes
- 2. There were 105 inspections undertaken in 2021/22
- 3. In 2021/22, 104 clinics were licensed by the HFEA to provide fertility treatment
- 4. No Grade A incidents since 2020/21, and severe OHSS remained consistent with previous years
- 5. Patient complaints decreased in 2021/22
- About our data
About this report
The Human Fertilisation and Embryology Authority (HFEA) is the independent regulator of fertility treatment and human embryo research in the United Kingdom (UK). We aim to ensure that everyone receives high quality care in a licenced fertility clinic. We do this by licensing, monitoring, and inspecting fertility clinics and providing free, clear and impartial information about fertility treatment, clinics, and egg, sperm, and embryo donation.
The ’State of the fertility sector’ is our annual report, which summarises what we have seen through our regulatory work during the year. The report is compiled from information gathered from our inspections and from other sources of information including our Register of fertility treatments, incident reports, and patient feedback mechanisms.
The COVID-19 pandemic had a significant impact on the availability of fertility treatment in 2020/21 and this, combined with the changes we made to our approach to inspection during the year, means the data provided in this report is not directly comparable with previous years and should be interpreted with caution. You can find out more about the HFEA regulatory response to COVID-19 and the subsequent changes made to inspections in the State of the fertility sector 2020/21 report, and the impact on treatment in the Impact of COVID-19 on fertility treatment 2020 report.
In 2019/20 we introduced Quarterly Clinical Governance Updates which provide clinics with detailed insight into non-compliances in a timely manner to help them improve compliance and prepare for inspections. These updates also contain details of incidents and complaints to ensure clinics can implement any recommendations to help prevent further recurrence. As a result, this State of the Sector report provides a high-level overview of sector-wide compliance, reporting on the absolute number of inspections, non-compliances, incidents, and complaints.
1. Changes to interim inspection themes
Treatment and storage centres usually receive a licence to operate for up to four years, although due to the COVID-19 pandemic some centres recently had their licence extended to five years, which is the maximum length of a treatment licence permitted according to the Human Fertilisation and Embryology Act 1990 (HFE Act 1990; as amended). For the HFEA to be satisfied that the centre can have its licence to provide treatment renewed, a full ‘renewal’ inspection would usually take place every four years. The HFE Act 1990 (as amended) also sets out a requirement that HFEA licensed clinics must have an on-site inspection every two years. Therefore an ‘interim’ inspection is carried out within two years of the renewal inspection in a standard four-year regulatory cycle. Interim inspections are most often unannounced (or sometimes with short notice), and only specific areas of practice are reviewed. The areas reviewed during interim inspections are highlighted by the interim inspection themes.
A review of the interim inspection themes was conducted during 2021, and the revised themes will be implemented during the inspection year 2022/23. The agreed themes were based on review of inspection reports and reported incidents from 2020. Data showing the breakdown of non-compliances which contributed to these decisions can be found in Figure 1 and in the underlying dataset. Details on which inspection themes will remain the same, be added, or removed can be found in Annex A; and further information can be found in our Quarterly Clinical Governance Updates.
The HFEA continues to focus on quality of care, patient safety and patient experience, in line with the HFEA Code of Practice (9th edition). Through the revised interim inspection themes the HFEA will provide ongoing assurance to patients, and improve compliance of UK licensed centres, in line with the HFEA strategy 2020-2023. This will continue to be assessed by review of clinical governance and effectiveness of centres’ quality management systems (QMS; detailed in Guidance note 23 of the HFEA Code of Practice (9th edition)).
Figure 1. Non-compliances identified in interim inspections influenced changes to interim inspection themes
Ten most common areas of non-compliances identified in interim inspections, 2020
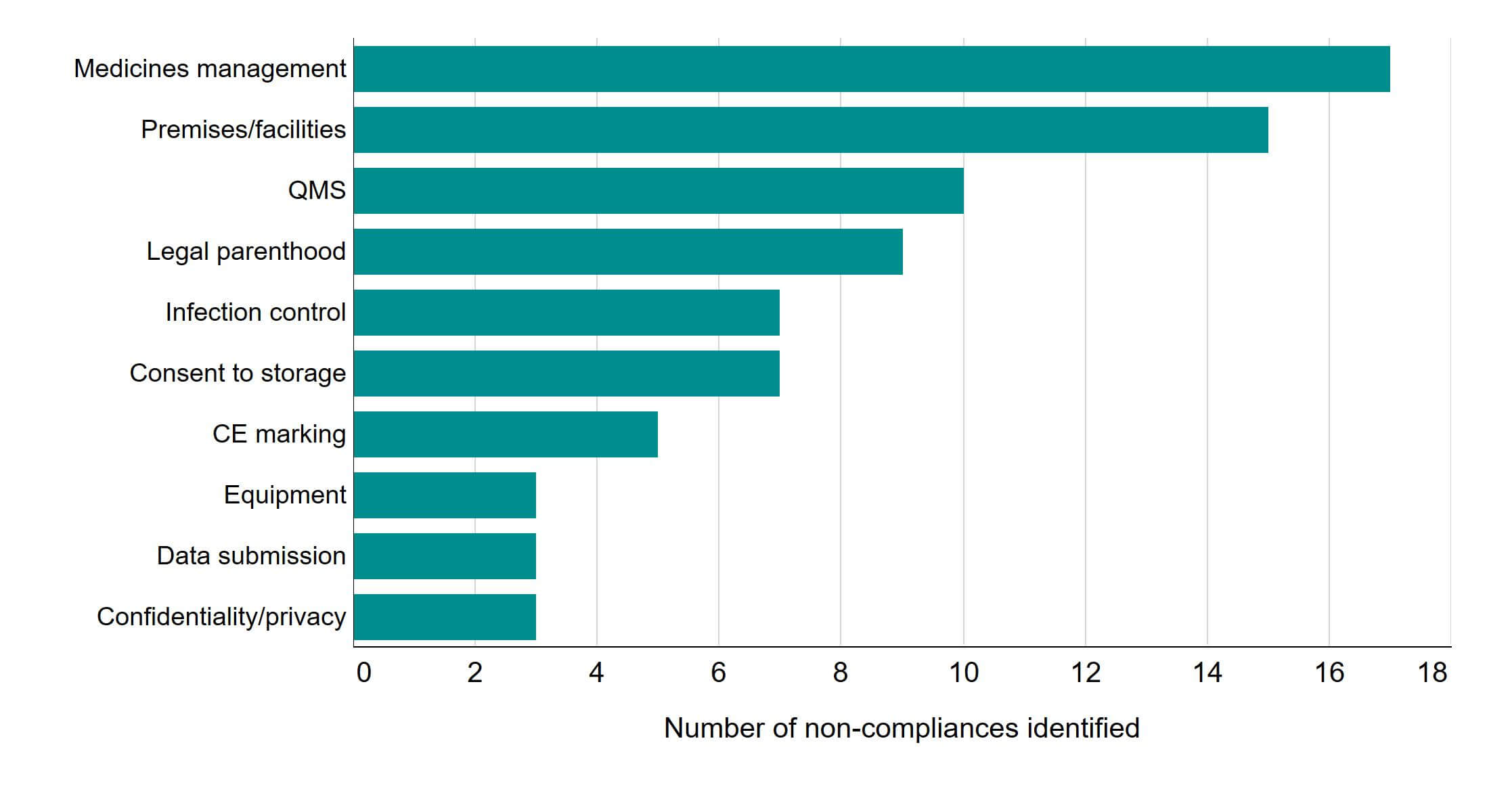
Note figure 1: These non-compliances were identified following 33 inspections carried out in 2020. Details on QMS can be found in Guidance note 23 of the HFEA Code of Practice (9th edition). There were also singular non-compliances identified during interim inspections in other areas, which are detailed in the underlying dataset.
Download the underlying data for Figure 1 as Excel Worksheet
2. There were 105 inspections undertaken in 2021/22
In 2021/22 clinics were assessed using a hybrid approach involving a desk-based assessment (DBA) combined with an onsite visit to allow continued close regulatory oversight of the fertility sector. A risk-based approach was taken in prioritising inspections due in this year with those deferred by the pandemic given priority. With interim inspections, this meant where the risk of non-compliance was assessed as low, the inspection was conducted without an onsite visit, often combined with a virtual inspection. More information on changes to inspections can be found in the State of the fertility sector 2020/21 report. The number of inspections increased from 2020/21 to 2021/22, which was primarily due to deferred inspections from 2020/21 during the COVID-19 pandemic.
There were 105 inspections in total carried out in 2021/22, of which:
- 63 were a combination of DBA and onsite visit
- 20 were onsite visits only
- 14 were a combination of DBA with virtual inspection
- Six were a combination of DBA, onsite visit, and virtual inspection
- Two were virtual inspections only
Figure 2 shows the number of inspections conducted since 2017/18, broken down by inspection type. Following each clinic inspection, a report identifying areas of good practice and those which are non-compliant and require improvement is published on the HFEA website. In 2021/22, 52 (50%) inspections were renewals, and 38 (36%) inspections were interim. There were also 15 renewal inspections which were deferred by extension of licence, and two voluntary revocations of licence, which are not included in this data. Additional inspections were conducted where significant concerns had been identified.
Figure 2. Inspections increased compared to previous years, following the COVID-19 pandemic
Number of inspections by type, 2017/18 – 2021/22
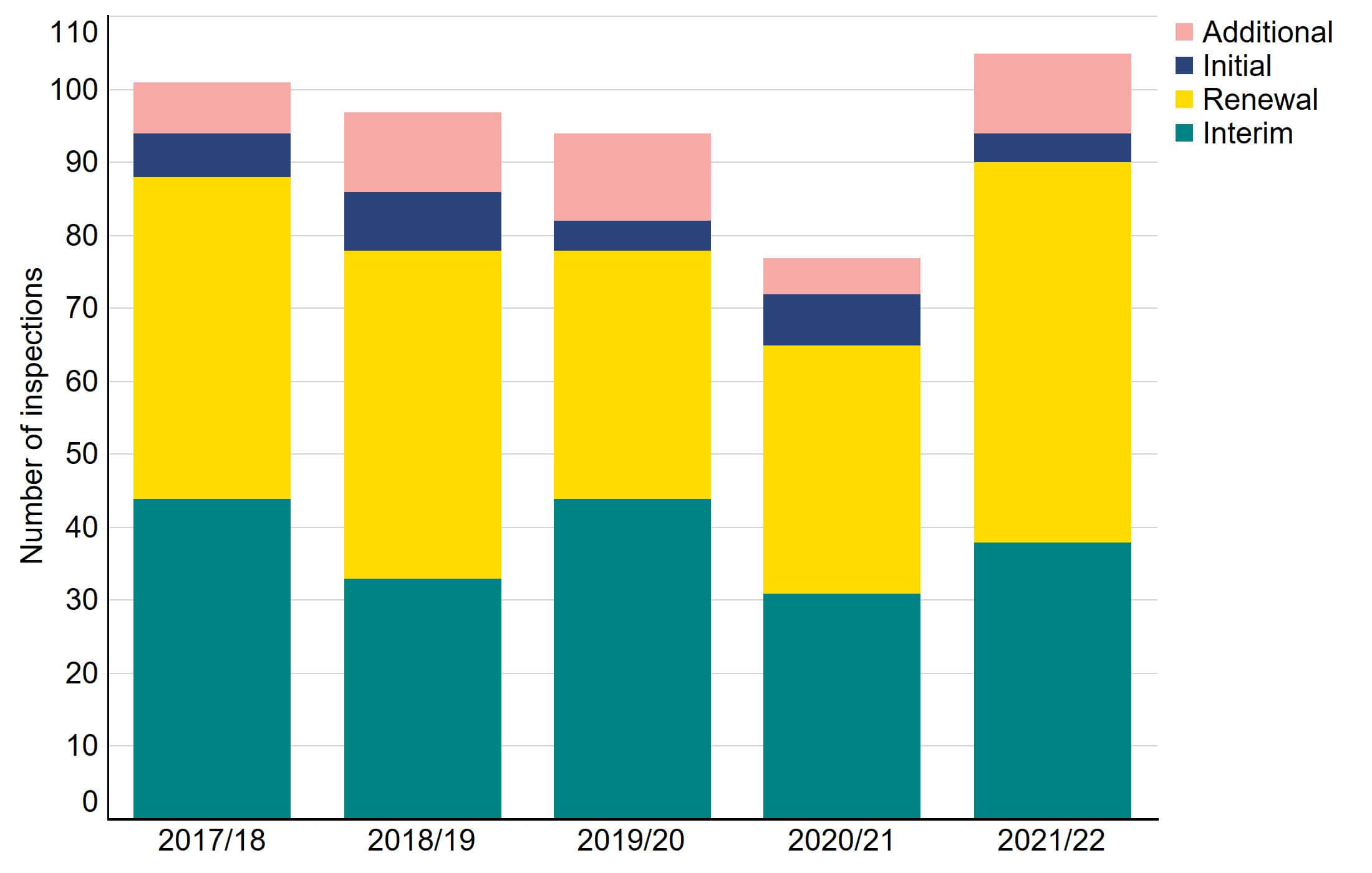
Note figure 2: Some inspections in 2020/21 were deferred until 2021/22 due to the COVID-19 pandemic.
Download the underlying data for Figure 2 as Excel Worksheet
In 2021/22, 231 non-compliances were identified during inspections, made up of:
- 107 Other i non-compliances
- 115 Major i non-compliances
- 9 Critical i non-compliances
As shown in Figure 3, only 4% of non-compliances were critical, highlighting the high quality of care provided by clinics in the UK. You can find out more about the types of non-compliances identified in 2021/22 in About this report and in the underlying dataset.
Figure 3. Only 4% of non-compliances were critical grade in 2021/22
Percentage of non-compliances by grade, 2021/22
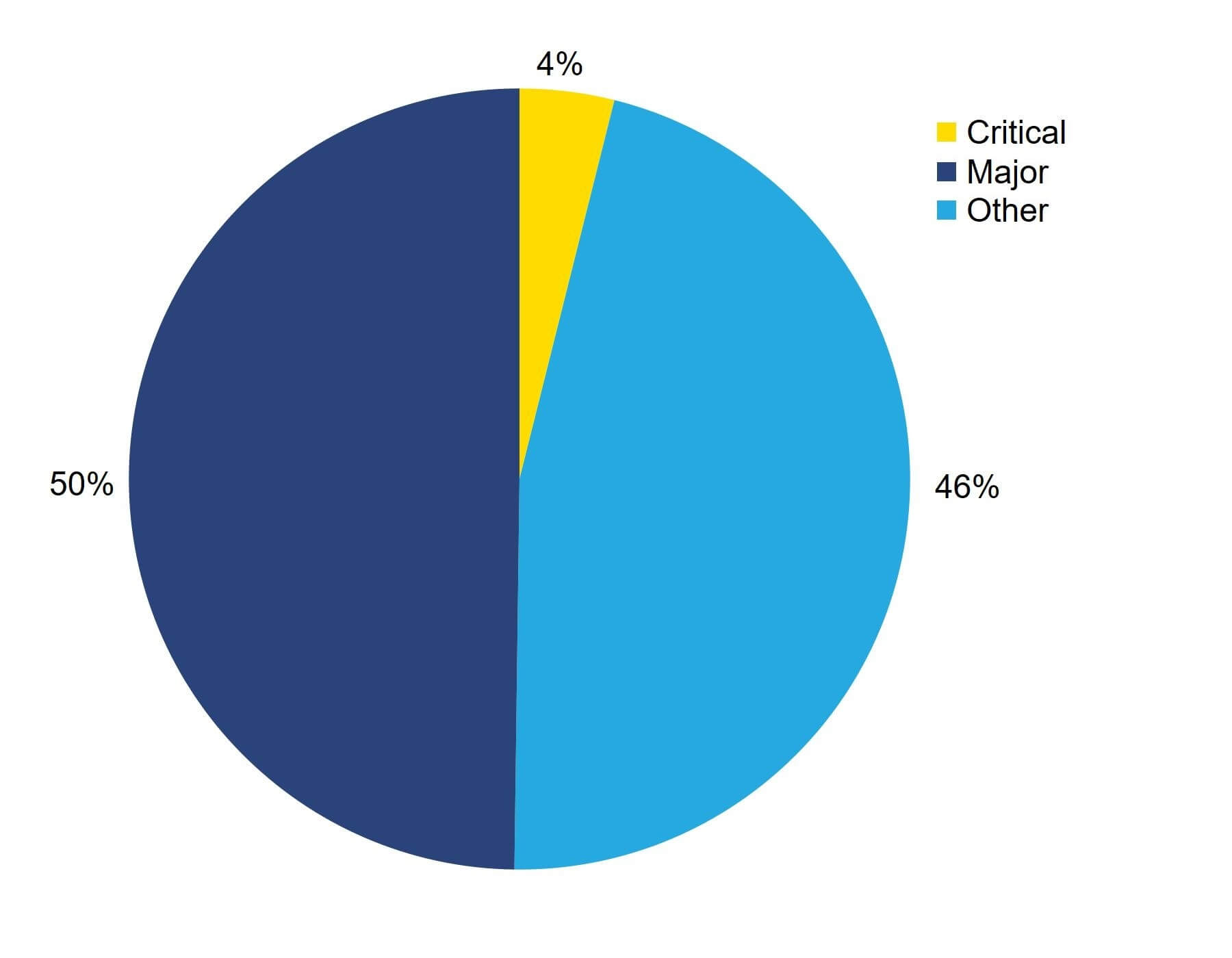
Note figure 3: This graph includes data from 231 non-compliances identified in 2021/22.
Download the underlying data for Figure 3 as Excel Worksheet
3. In 2021/22, 104 clinics were licensed by the HFEA to provide fertility treatment
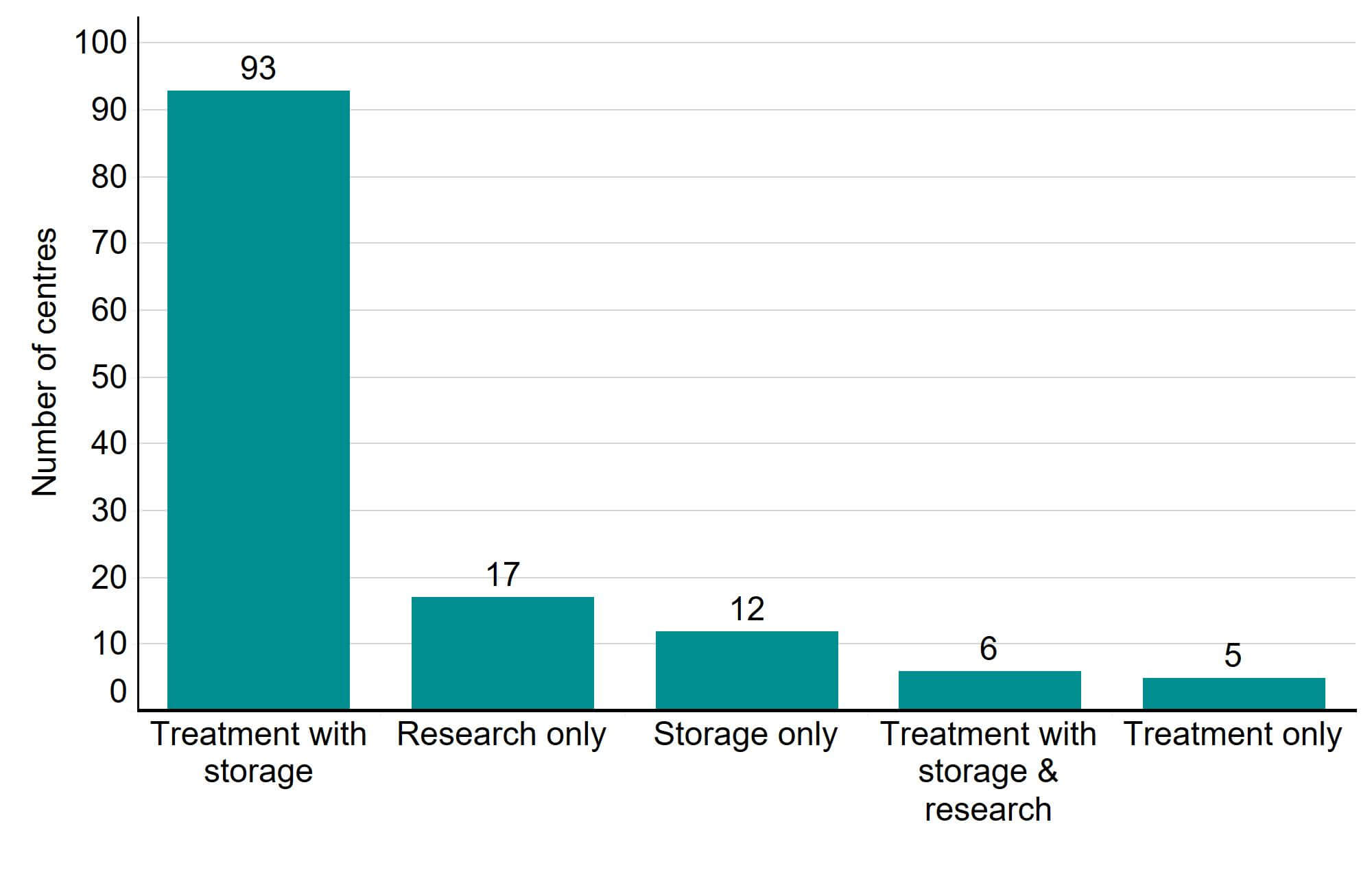
In 2021/22 there were 104 fertility clinics licensed by the HFEA to provide fertility treatment in the UK. As seen in Figure 4, an additional 17 establishments were licensed to undertake research and 12 were licensed to provide storage only.
Of the 104 licensed treatment clinics, 62 (60%) were privately owned, an increase from the 59 in 2020/21. It should be noted that most HFEA licensed clinics, whether private or NHS, treat both NHS and privately funded patients.
As shown in Figure 5, the largest concentration of clinics offering fertility treatment was based in London (33 clinics), followed by the North West and South East (10 clinics each). Northern Ireland currently has the smallest number of treatment clinics, which had decreased from four to three since 2020/21 following the closure of a small IUI clinic.
Figure 4. The majority of HFEA licensed clinics provided both treatment and storage to their patients
Number of licensed fertility clinics by type, 2021/22
Download the underlying data for Figure 4 as Excel Worksheet
Figure 5. London continued to have the highest number of HFEA licensed fertility clinics
Number of clinics licensed to provide fertility treatment by geographical area, 2021/22 (excluding storage and research only)
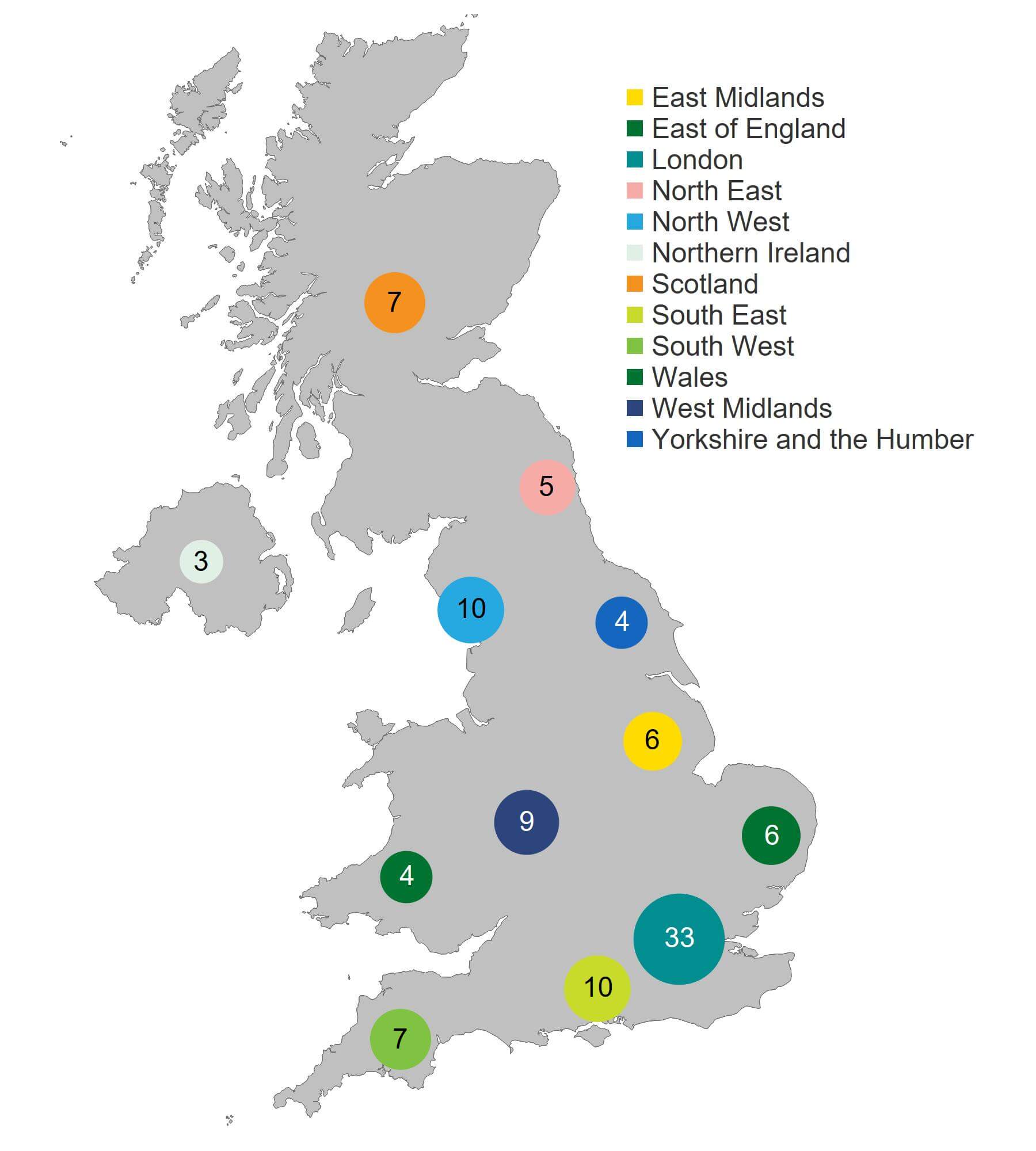
Download the underlying data for Figure 5 as Excel Worksheet
4. No Grade A incidents since 2020/21, and severe OHSS remained consistent with previous years
Almost 90,000 treatment, storage and donation cycles are carried out each year ii, and over 99% of these are carried out without any incidents being reported. Of those incidents, very few are of the most serious type – Grade A iii. Clinics and the HFEA take adverse incidents very seriously, and we have a rigorous process for reporting, handling, and investigating adverse incidents and near misses. Reporting adverse incidents in IVF is a statutory requirement but it is also recognised as one of the best ways of ensuring that incidents and their causes are identified. This process allows clinics to learn from incidents and ensure changes can be made to prevent reoccurrence. We monitor incidents in clinics to make sure that everything is done to understand what went wrong and, crucially, to take steps to ensure it does not happen again. We also share learning and notify other clinics of potential issues to ensure all clinics learn from this. The Quarterly Clinical Governance Updates provide clinics with detailed insight into incidents.
There were 793 incidents (including 121 near misses) reported to the HFEA in 2021/22, compared to 548 in 2020/21. This increase is due to the requirement for clinics to report all patient hospital admissions due to the COVID-19 pandemic. Grade B incidents remain consistent with previous years, and no Grade A incidents occurred in 2021/22 or in 2020/21.
Ovarian Hyperstimulation Syndrome (OHSS) is a potentially serious side effect of the drug treatment necessary for fresh cycles of IVF, which unfortunately some patients develop, as shown in Figure 6. We have worked hard with clinics to ensure they do everything possible to prevent and manage OHSS. OHSS can range in severity from mild to critical, and is graded by the patient’s clinic in accordance with guidelines by the Royal College of Obstetricians and Gynaecologists. All cases of severe or critical OHSS must be reported to the HFEA within 24 working hours by the patient’s clinic. During the COVID-19 pandemic clinics were required to report all hospital admissions (including mild/moderate OHSS) to reduce the increased burden on the NHS. In February 2022, we notified all clinics that this reporting was no longer required.
In 2021/22 there were 66 cases of severe and critical OHSS reported by UK clinics, occurring in less than 0.1% of cycles.
Figure 6. Severe and critical OHSS incidents remain consistent with previous years
Number of severe and critical OHSS incidents reported 2017/18 – 2021/22
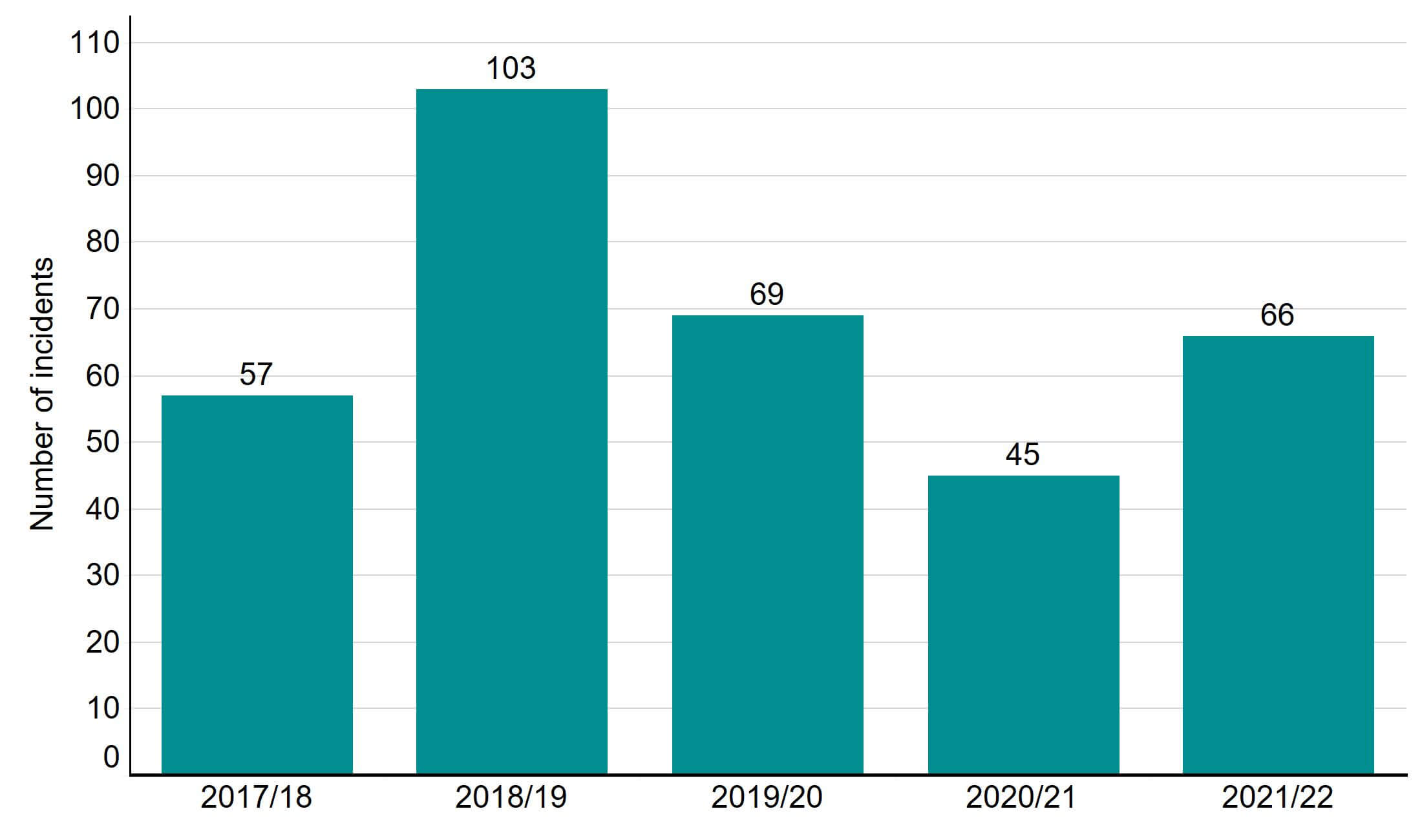
Download the underlying data for Figure 6 as Excel Worksheet
5. Patient complaints decreased in 2021/22
The HFEA does not have a statutory duty to investigate patient complaints, unless the patient complaint describes a serious breach of the HFE Act or Licence Conditions.
However, patients and donors can raise a complaint with us if they are unhappy with how a clinic has handled their original complaint. We actively monitor and investigate the information we receive regarding patient complaints and feed this into the inspection process. The Competition and Markets Authority (CMA) has launched guidance on fertility treatment for clinics and patients.
Where a complaint is received, we discuss it with the clinic and encourage them to further engage with the complainant to try to locally resolve the issues raised. Complaints are discussed at regular clinical governance meetings to ensure clinics are actively engaging with patient complaints and providing thorough and well considered responses. We expect clinics to provide an honest acknowledgement of the complainant’s experience, to explain what, if anything, went wrong, what measures the clinic has put in place to minimise the risk of this happening again, and what support the clinic can offer the complainant. The Quarterly Clinical Governance Updates provide clinics with detailed insight into complaints.
The number of patient complaints received in 2021/22 was 76, which has decreased since the previous year, as shown in Figure 7. Around 60,000 patients undergo treatment, storage, and/or donation cycles per year. About 0.1% of these patients submit complaints to the HFEA annually. This may be in part due to continued HFEA support and guidance to ensure that wherever possible clinics engage with the complaint to resolve the problem before there is an escalation to a formal complaint. This guidance continues to be relevant as complaints received by the HFEA in 2021/22 often related to patients wanting greater engagement from their clinic about their original complaint.
Patient complaints continue to be complex. Often raising concerns about care, communication and lack of satisfactory response to an initial complaint. Most patients who bring their complaints to us want the clinic to learn from their experience and put measures in place to ensure other patients do not go through a similar situation, and for the clinic to offer an apology when necessary. Further advice to clinics on engaging and resolving patient complaints was included in our State of the Sector 2018/19 report and we urge clinics to incorporate this into their complaint handling processes.
Figure 7. The number of complaints received has decreased since 2020/21
Number of complaints received, 2017/18 - 2021/22
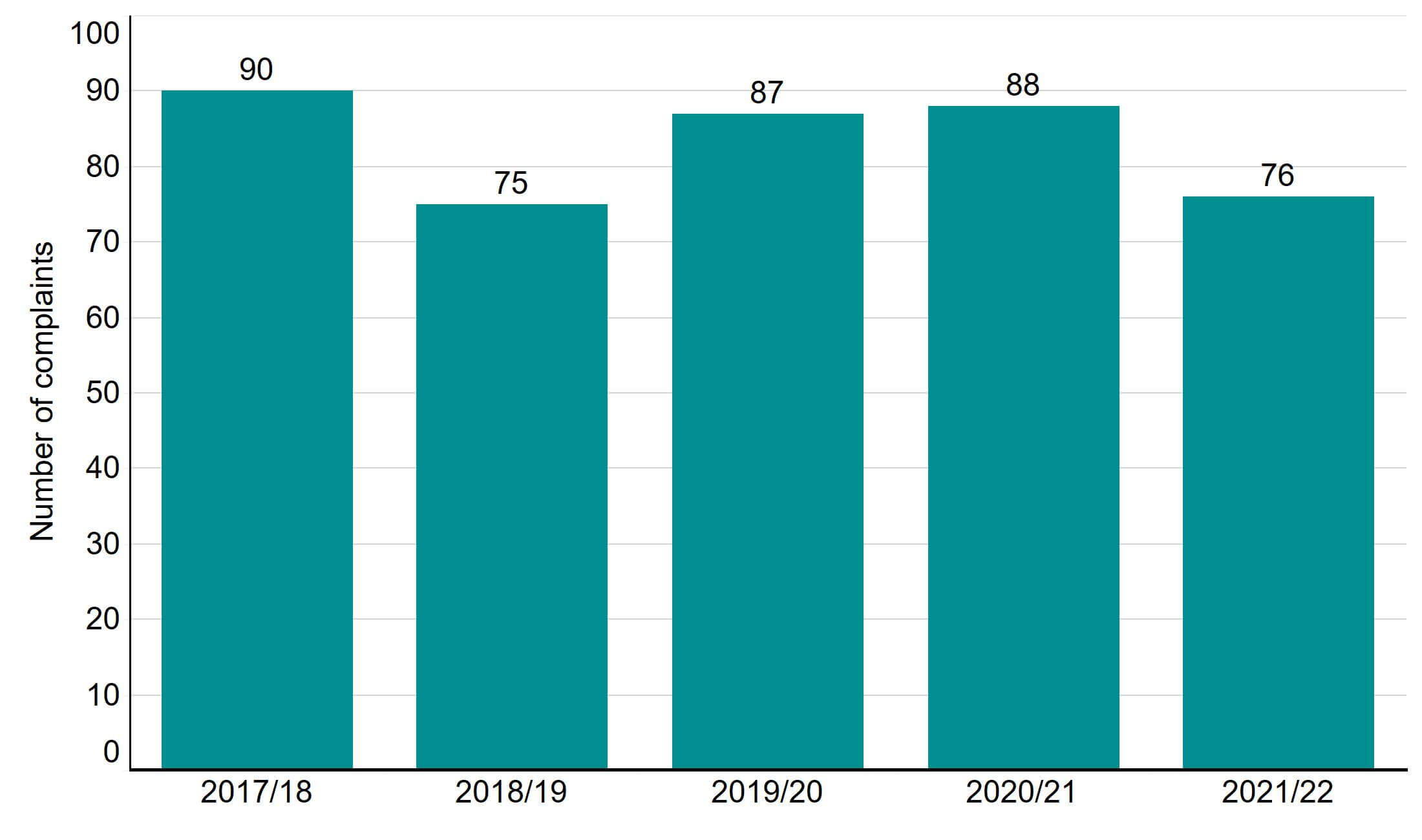
Download the underlying data for Figure 7 as Excel Worksheet
About our data
The information that we publish in this report is compiled from information gathered from our inspections throughout the year and other sources of information including our Register of fertility treatments, incident reports and patient complaints. A full list of definitions of classification of incidents and complaints are available in the underlying dataset.
Contact us regarding this publication
Media: press.office@hfea.gov.uk
Statistical: intelligenceteam@hfea.gov.uk
Accessibility: comms@hfea.gov.uk
Notes on State of the Sector 2021/22
i Non-compliances are graded as:
- Critical: an area of practice which poses a significant risk of causing harm to a patient, donor, embryo or to a child who may be born as a result of treatment services, or a significant shortcoming from the statutory requirements
- Major: an area of practice which poses an indirect risk to the safety of a patient, donor, embryo or to a child born as a result of treatment services. This area of non-compliance may also indicate a major shortcoming from the statutory requirements and/or indicate a failure by the Person Responsible to carry out their legal duties.
- Other: an ‘other’ area of practice that requires improvement is any area of practice, which cannot be classified as either a critical or major area of non-compliance, but which indicates a departure from statutory requirements or good practice.
ii In 2019 there were Approximately 90,000 cycles of treatment/storage/donation carried out:
- Approximately 76,500 treatment cycles (69,000 IVF; 5,500 DI; 2,000 Genetic testing)
- Approximately 10,500 Storage cycles
- Approximately 2,000 Donation cycles
iii Incidents are graded as:
- Grade A: involve severe harm to one person, or major harm to many
- Grade B: involves serious harm to one person, or moderate harm to many
- Grade C: involves minor harm
- Near miss: an event not causing harm, but has the potential to cause injury or ill health.
Annex A
Inspection themes to be retained
- Pre-inspection review of data quality. The registry team provide a report to the inspection team, highlighting any reporting issues regarding treatments reported to the registry.
- Pre-inspection review of the centre’s history of compliance, Risk Based Assessment Tools (RBATs), patient questionnaire reports, incidents and complaints and centre’s websites. The clinic’s compliance history will be reviewed during the pre-inspection analysis. If there are any significant areas of concern which are not covered by the interim inspection themes, the Inspector will follow-up on these during the inspection.
- Pregnancy outcomes and multiple pregnancy rates. This is a fundamental aspect of the HFEA strategy to ensure patients receive safe and effective care.
- Patient safety, feedback & emotional support. This is a key part of the HFEA strategy. This is assessed using ‘Choose a Fertility Clinic’ patient feedback, the clinic’s patient surveys, patient support policies, and patient interviews during the inspection.
- Leadership, staffing & clinical governance. This is a newer area of focus in the HFEA strategy and current Code of Practice. This will be assessed through the overall findings of the inspection, the clinic’s governance processes, and the PR’s oversight, and will address any staffing concerns raised.
- Quality Management System (QMS). This continues to be one of the most common areas of non-compliance reported. The Inspector will focus on the effectiveness of the clinic’s QMS, their implementation of recent regulatory changes, their lessons learned from incidents (internal, published grade A), and their actions on updates (e.g., new legislation, Chair’s letters, Chief Executive letters, Clinic Focus, professional bodies).
- The Inspector will review centre’s own audits of consent (storage, legal parenthood), medicines management, and infection control. The HFEA will perform audits of practice if areas of concern are identified and/or are considered necessary during the inspection.
- Surgical procedures. Recent inspection evidence highlighted several aspects of surgical procedures as risk areas, including management of medicine, infection control, and the clinic premises.
Themes to be added
- Previously only consent to storage was considered in the HFEA interim inspection themes, however this is to be expanded to include wider consent practices. Consent is one of the most common areas of non-compliance identified, and all areas of consent will be considered in addition to consent to storage. This will be assessed in conjunction with the centre’s own audits. As new storage legislation came into force on 1 July 2022 inspectors will focus on clinic understanding of, and compliance with the new law.
- Donor recruitment, selection, assessment, and screening. This was a common area of non-compliance identified during inspection. Clinics should continue to complete the HFEA audit tool. Inspectors can use this to audit records during the inspection should any concerns be raised.
Current interim inspection themes to be removed
- Consent to storage. This is to be replaced with a focus on wider consent practices, not just storage consent (see the ‘Themes to be added’ section above).
- Whilst a very important area of practice, the process of witnessing has not been identified as a significant concern during the most recent review of compliance evidence from inspections 2019-2020.
- Review centre’s audit of legal parenthood in response to Chief Executive’s letter CE(14)01. This audit has now been reviewed at all centres. Whilst this particular requirement has been removed all clinics are still required to perform routine audits of consent to legal parenthood. These audits are submitted and assessed during the inspection.
- CE marking of medical devices. Recent inspection evidence suggests the fertility sector has a good level of compliance regarding the use of CE marked medical device products.
Review date: 15 April 2027

