The fertility sector 2024/25
Annual publication on HFEA licensed clinics (1 April 2024 – 31 March 2025)
Published: November 2025
Download the underlying dataset as Excel Worksheet
Table of contents
- 1. How we regulate
- 2. Main points
- 3. How the HFEA inspects clinics
- 4. Inspections
- 5. Non-compliances identified at inspections
- 6. Clinic licences
- 7. Incidents
- 8. Ovarian Hyperstimulation Syndrome (OHSS)
- 9. Patient complaints
- 10. Patient feedback
- 11. About our data
1. How we regulate
The Human Fertilisation and Embryology Authority (HFEA) is the independent regulator of fertility treatment and human embryo research in the United Kingdom (UK). We aim to ensure that everyone who steps into a licensed fertility clinic receives high quality care and we do this by licensing, inspecting and setting standards. We also provide free, clear and impartial information about fertility treatment, clinics, and egg, sperm, and embryo donation.
This year, our Fertility Trends 2023 report showed that 1 in every 32 UK births is from IVF, or around one child in every classroom. There is, understandably, strong public interest in the safety and effectiveness of fertility treatment, and in several different aspects of the HFEA’s regulatory remit.
Our ‘The Fertility Sector 2024/25’ report (previously known as ‘State of the sector’) is a summary of what we have seen through our regulatory work during the year.
Having treatment in a UK licensed fertility clinic continues to be very safe. This year, more than 100,000 cycles of fertility treatment, and storage or donation took place in UK clinics, with incidents occurring in less than 1% of cycles. In addition, the HFEA’s National Patient Survey, published in March 2025, found that almost three-quarters of patients were satisfied with their latest round of treatment.
Although incidents are rare, each one may be upsetting for the patients involved. There has been an overall increase in the number of incidents reported to the HFEA over the last year. One reason for the increase could be that following high-profile incidents in two clinics in 2023/24, clinics are responding to the learning shared by the HFEA by more diligently reporting incidents. As in other healthcare settings, the reporting of incidents is a sign of a responsible sector and we will continue to monitor trends over time and ensure that any learnings from incidents is shared and acted upon.
The largest increase in incidents has been in the lowest grade - Grade C. There is a more detailed explanation of what this means in Section 7 of this report, but it includes a rise in clinic administration incidents. We know from our National Patient Survey the impact these have on patients, so we are raising this as an area where clinics can improve.
There has also been a high level of interest in egg donation in the last year. The process of donating eggs is generally very safe. However, clinics must do everything possible to prevent and manage any risks, including Ovarian Hyperstimulation Syndrome (OHSS) where clinics are required to report severe and critical cases as an incident. In the last year, none of the cases of severe and critical OHSS reported to the HFEA in 2024/25 related to egg donors.
There has also been strong public interest in the safety of those using private sperm donors. The HFEA emphasises that it is always safer for those having fertility treatment to use a HFEA-licensed clinic, where there are laws and guidance to protect and support patients and donors.
The fertility sector continues to change. The number of self-funded patients in the UK continues to rise, with our most recent report showing 27% of cycles in the UK are NHS-funded, a drop from 35% in 2019 . Independent services are growing in number and may include patients completing their pre-treatment information, counselling, tests and scans through mobile apps, concierge services or via independent healthcare hubs, rather than at a licensed fertility clinic. Only HFEA licensed clinics can carry out those aspects of treatment requiring a licence, such as using eggs and sperm to create embryos and storing eggs, sperm and embryos.
This can be challenging from a regulatory perspective. It might not always be clear to patients whether a service is regulated by the HFEA or not. Patients may be under the false impression that a service provider which, for example, has taken their consent, is regulated by the HFEA, when this may not actually be the case, and if this service closes then the HFEA cannot help patients nor carry out enforcement action.
This challenge was brought into sharp focus following the high-profile closure in December 2024 of Apricity, a non-HFEA licensed virtual ‘clinic’, and the resulting concern and distress caused to patients who were planning treatment - some of whom reported losing thousands of pounds. The HFEA’s more than 30-year-old regulatory powers are very limited in these circumstances, as we cannot carry out enforcement action on services that are outside the Human Fertilisation and Embryology Act. Our effective regulatory tools, therefore, are the inspection and licensing of clinics.
2. Main points

- In 2024/25, the HFEA completed 88 inspections, a decrease from 104 in 2023/24. This is because of a change to the length of licences issued during the COVID-19 pandemic.
- 131 non-compliances were identified during inspections in 2024/25, down from 226 in 2023/24. This decrease relates in large part to the lower number of inspections carried out in 2024/25 compared to the previous year. Across all clinics, however, the average number of non-compliances was 1.5 per inspection compared with around 2.2 non-compliances per inspection in the previous year.
- The total number of clinics licensed by the HFEA in 2024/25 was 141, an increase of six since 2023/24. Of these, 107 are licensed to provide fertility treatment with the remainder licensed to store gametes and embryos, or for research.
- There were 792 incidents reported to the HFEA in 2024/25, 36% more than the 581 incidents reported in 2023/24. Despite the increase, this means more than 99% of cycles that took place at UK licensed clinics did so without an incident.
- In 2024/25, UK licensed clinics reported 67 cases of severe and critical OHSS.
3. How the HFEA inspects clinics
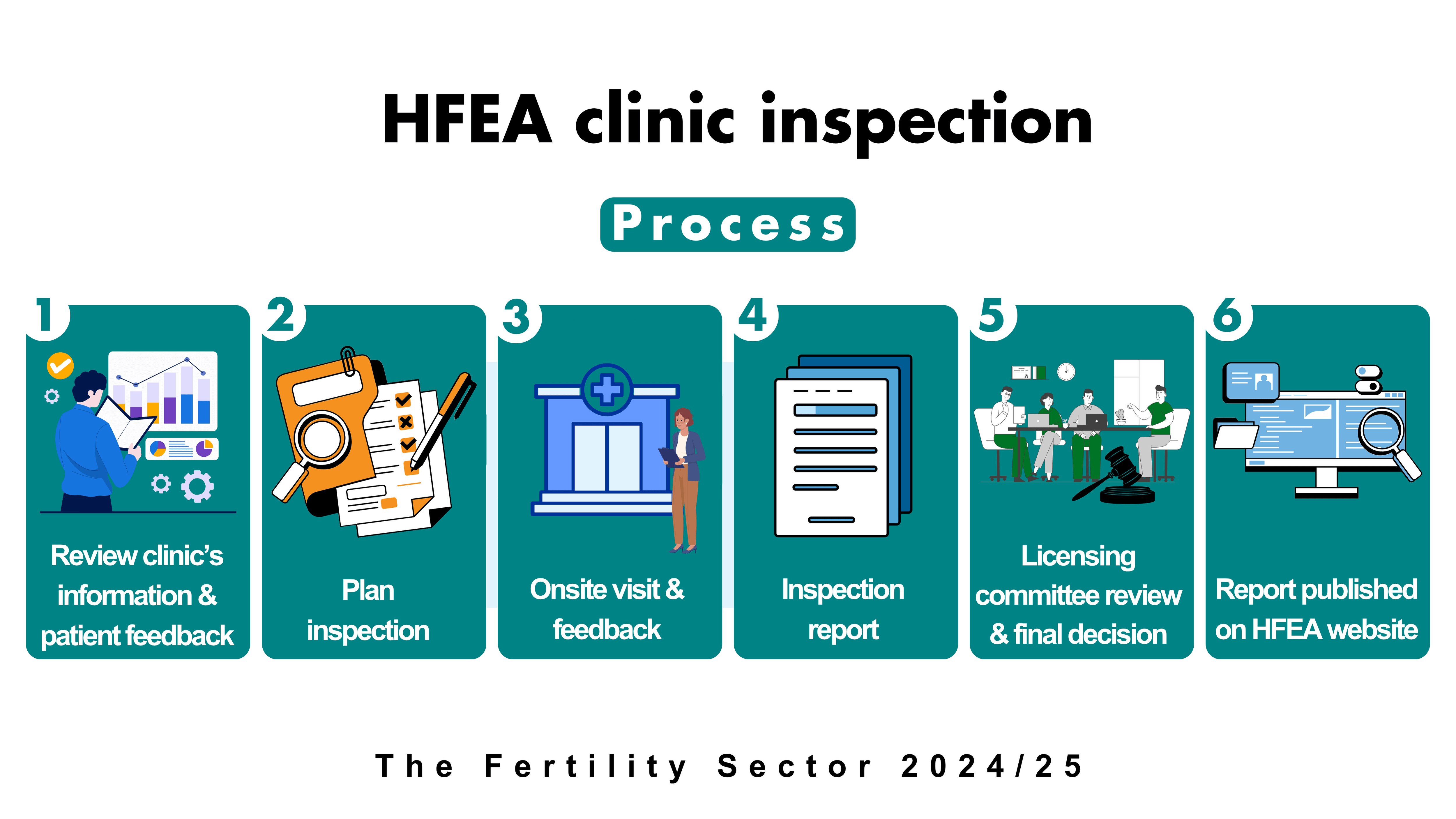
Clinics are inspected through a combination of reviewing information in advance of an onsite visit, and the visit itself. This approach is robust and efficient, allowing the inspection to focus on specific concerns and areas of practice which are better assessed on site.
By law every clinic must be physically inspected every two years. The HFEA has both clinical and scientific inspectors, who inspect areas specific to their knowledge and expertise. Each inspector oversees a portfolio of clinics, which allows knowledge to be built up around each one, as well as good working relationships with the clinic.
Inspectors inspect clinics but do not make the final decision on licensing. Following each inspection, a report identifying both areas of good practice and those that require improvement is presented to a separate licensing committee, to review and make a final decision. This is important because the only regulatory sanction available to the HFEA is the power to issue, suspend, impose conditions on a licence or revoke a clinic’s licence.
The report and committee decision is then published on the clinic’s individual Choose a Fertility Clinic page on the HFEA website, where it is available for anyone to read.
The recommendations from HFEA inspectors support clinics to make improvements to reach the standards expected of them within an agreed timeframe. Further action, such as suspending a clinic’s licence or closer oversight, such as regular or unannounced inspection visits, can be taken if these improvements are not made.
In accordance with the Public Interest Disclosure Act 1998 (PIDA) all whistleblowing allegations and concerns raised with the HFEA are investigated. Individuals are protected by PIDA and are treated confidentially. Any current or former clinic staff member can raise whistleblowing concerns or allegations by visiting the HFEA website or using the dedicated whistleblowing email address. Inspectors, while on inspection, also leave leaflets in clinics with details on how to raise a concern.
4. Inspections 2024/25
The HFEA carried out 88 inspections in 2024/25, a decrease from 104 in 2023/24 (Figure 1). This is because of a change to the length of licences issued during the COVID-19 pandemic. There were three initial inspections and 24 renewal inspections. Of the 55 interim inspections in 2024/25, there was one announced, one short notice, and five unannounced focused inspections.
Six additional inspections were carried out in 2024/25, however these related to variations and changes in premises, rather than as a result of major concerns.
Figure 1. There were 88 inspections undertaken in 2024/25
Number of inspections by type, 2020/21-2024/25
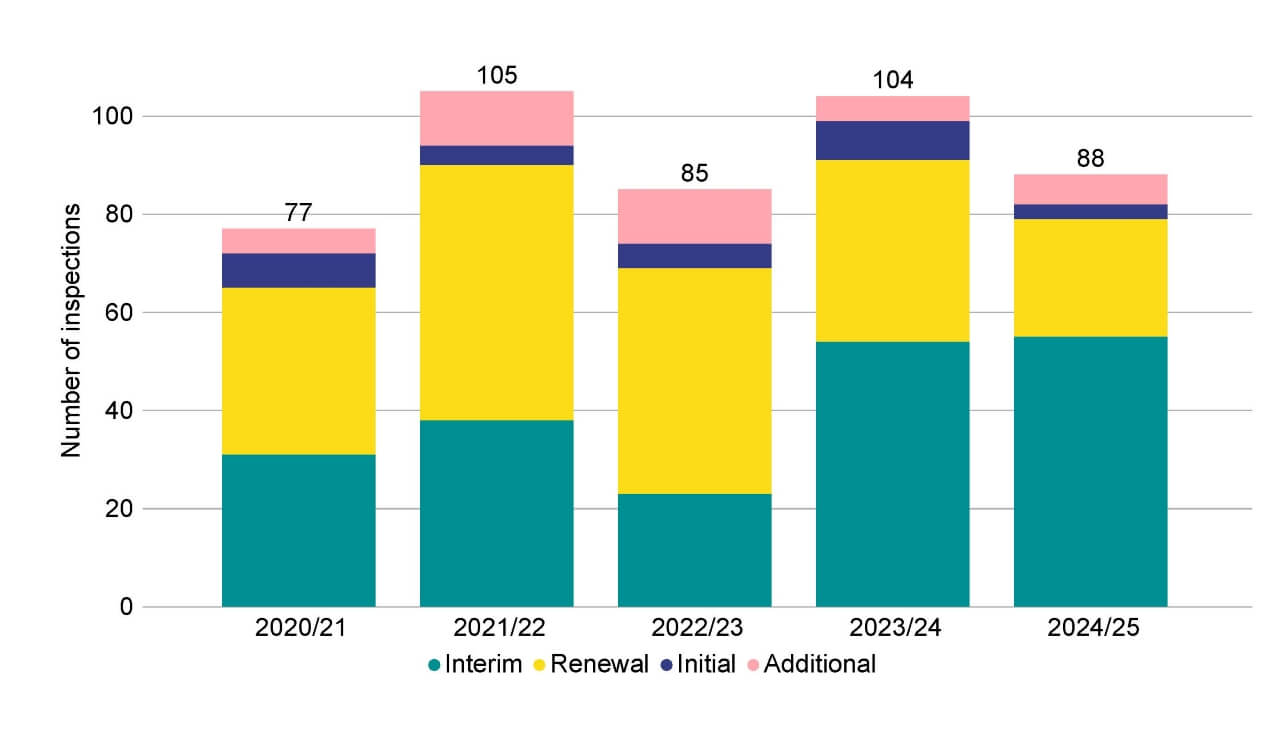
Note figure 1: Interims included one announced interim, one short notice interim, five unannounced focused interim inspections. Additional inspections included five variation of premises, and one change of premises.
Download the underlying data for Figure 1 as Excel Worksheet
In 2024, the effectiveness of regulation came under the spotlight, with the publication of independent reports on the CQC and Ofsted. The HFEA reviewed our own processes in light of these reports.
While aspects of the CQC’s and Ofsted’s approach and responsibilities are different from the work of the HFEA, there was much in the reports which was relevant and allowed us to analyse where our strengths and weaknesses lie and where we have opportunities to improve.
The HFEA inspection regime has undergone significant change in the last few years including a new Compliance and Enforcement Policy and introduction of a hybrid approach to inspection (utilising desk based analysis and on-site visits). These changes were independently audited by the Government Internal Audit Agency (GIAA) on two separate occasions (March 2021 and April 2022) and the regime as a whole was independently assessed by the Public Body Review of the HFEA in 2023.
Looking at the reviews of the CQC and Ofsted, we judged that it is clear where our strength lies: the expertise of the inspectors and clinical governance team, a robust regulatory regime which ensures clinics are inspected in a defined timeframe and that Persons Responsible (PRs) have a named inspector to communicate with. We have strong oversight from our board (the Authority) and are held accountable by our sponsorship team at the Department of Health and Social Care (DHSC).
A learning point raised in the review of Ofsted was that the essence of a good inspection is respectful and productive engagement between inspectors and those inspected. Direct feedback from clinics helps us to gauge whether this is the case for HFEA inspections. Inspectors underwent training on recognising stress during inspections and dealing empathetically with situations.
After every inspection the PR receives a questionnaire regarding the entire inspection process. We strongly encourage PRs to complete these post-inspection surveys.
Between April 2024-March 2025, 85% of respondents to the post-inspection survey agreed that clinics were given the opportunity to discuss and understand inspection findings and areas for improvement during the inspections team feedback, and 75% of respondents agreed that the findings within the inspection report were accurate and clearly presented. Each inspection report gives the opportunity for the PR to provide feedback if they want to raise concerns over how the findings have been presented.
5. Non-compliances
Since 2021/22, the average number of non-compliances identified at inspections has remained consistent at 2.2 non-compliances per inspection, however in the last year (2024/25) this has decreased to 1.5 non-compliances per inspection overall.
More than a third (35%, 21) of clinics with treatment or storage licences that were inspected in 2024/25 had no non-compliances. In those clinics where non-compliances were identified, the average number was 2.2.
While the HFEA welcomes the decrease, we will continue to monitor this trend in future years.
Non-compliances are graded as ‘critical’, ‘major’ or ‘other’. A critical non-compliance can include, for example, staffing levels not being suitable to carry out licensed activities. Major non-compliances can include, for example, patients having gametes and embryos in storage but consent to storage had expired.
In the last year, 131 non-compliances were identified during inspections (Figure 2). Of these non-compliances, 63 were identified as ‘major’, 62 ‘other’, and six ‘critical’. This decreased from 226 in 2023/24. This 42% decrease will relate in large part to the lower number of inspections carried out in 2024/25 compared to the previous year. The proportion of critical non-compliances has increased from 3% in 2023/24 to 5% in 2024/25, but remains at six critical non-compliances overall, the same as 2023/24.
Figure 2. There were 131 non-compliances identified at inspection in 2024/25
Number of non-compliances by grade, 2020/21-2024/25
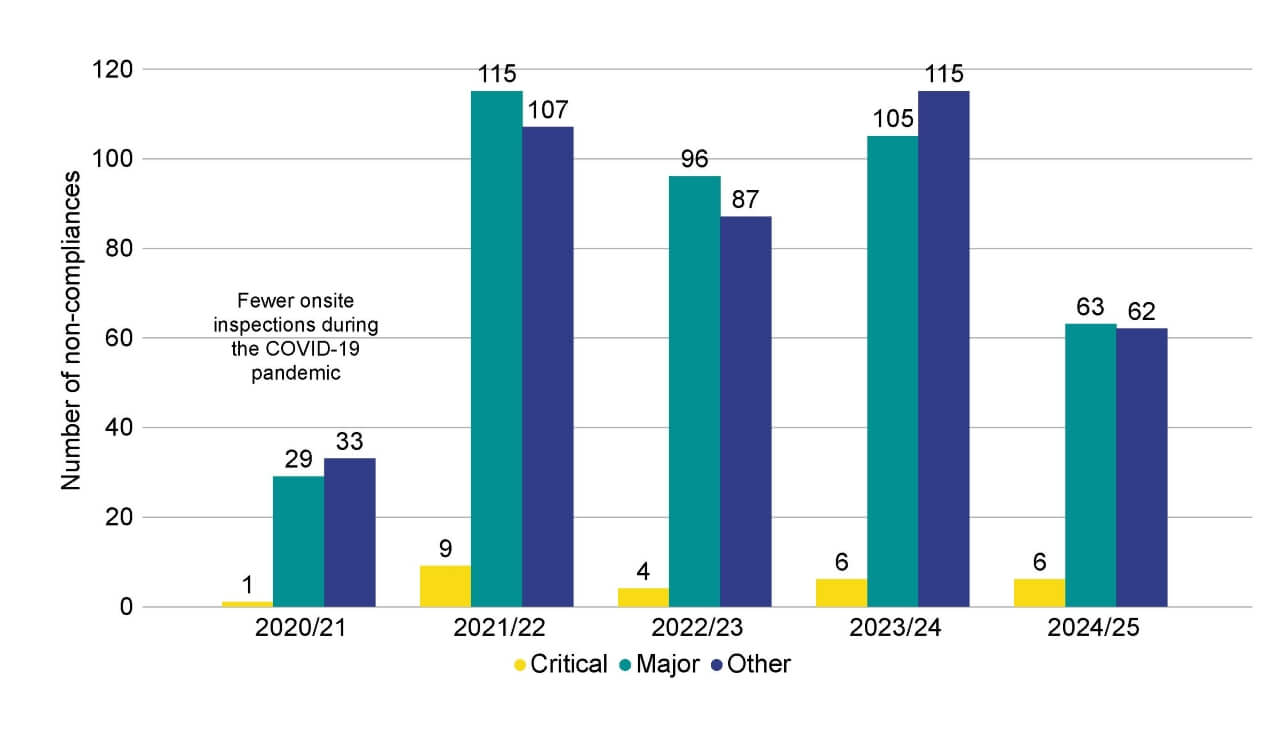
Note figure 2: Inspections in 2020/2021 were impacted by COVID-19 measures introduced by the HFEA. This data includes non-compliances identified at treatment and storage clinics, and excludes non-compliances identified at research only clinics.
Download the underlying data for Figure 2 as Excel Worksheet
6. Clinics licensed in 2024/25
The fertility sector is changing. Several establishments have appeared in recent years that describe themselves as fertility clinics, and patients beginning treatment may now face a fragmented journey with a combination of online and in-person licensed and independent services.
It might not always be clear to patients whether a service is regulated by the HFEA or not. Patients may believe that a service provider which, for example, has taken payment or taken their consent, is regulated by the HFEA, when this may not be the case.
A service may use multiple physical services throughout the treatment pathway, for example, use a premise which offers scanning, use online consenting platforms and then refer patients, or ‘satellite’ to a licensed clinic for treatment. Only HFEA licensed clinics can carry out those aspects of treatment requiring a licence, such as using eggs and sperm to create embryos and storing eggs, sperm and embryos.
The total number of clinics licensed by the HFEA in the last year was 141, an increase of six since 2023/24.
In 2024/25 there were 107 fertility clinics licensed by the HFEA to provide fertility treatment in the UK (Figure 3). A further 19 establishments were licensed to undertake research and 15 were licensed to provide storage only.
Of the 107 licensed treatment clinics, 71 (66%) were privately owned, an increase from 62 in 2021/22. It should be noted that most HFEA licensed clinics, whether private or NHS, treat both NHS and self-funded patients. Of the 71 private clinics, 22 were standalone, and the remaining 49 were owned by clinic groups. Further information about clinics is available in the underlying dataset and Choose a Fertility Clinic.
The region with the largest number of clinics carrying out treatment was in London (36 clinics), followed by the South East (10 clinics). Northern Ireland and Wales had the fewest treatment clinics at three and four respectively.
Figure 3. Most clinics are licensed by the HFEA for treatment and storage
Number of licensed clinics by licence type, 2024/25

Download the underlying data for Figure 3 as Excel Worksheet
Figure 4. London had the highest number of HFEA fertility clinics licensed for treatment
Number of fertility clinics licensed for treatment by geographical area, 2024/25
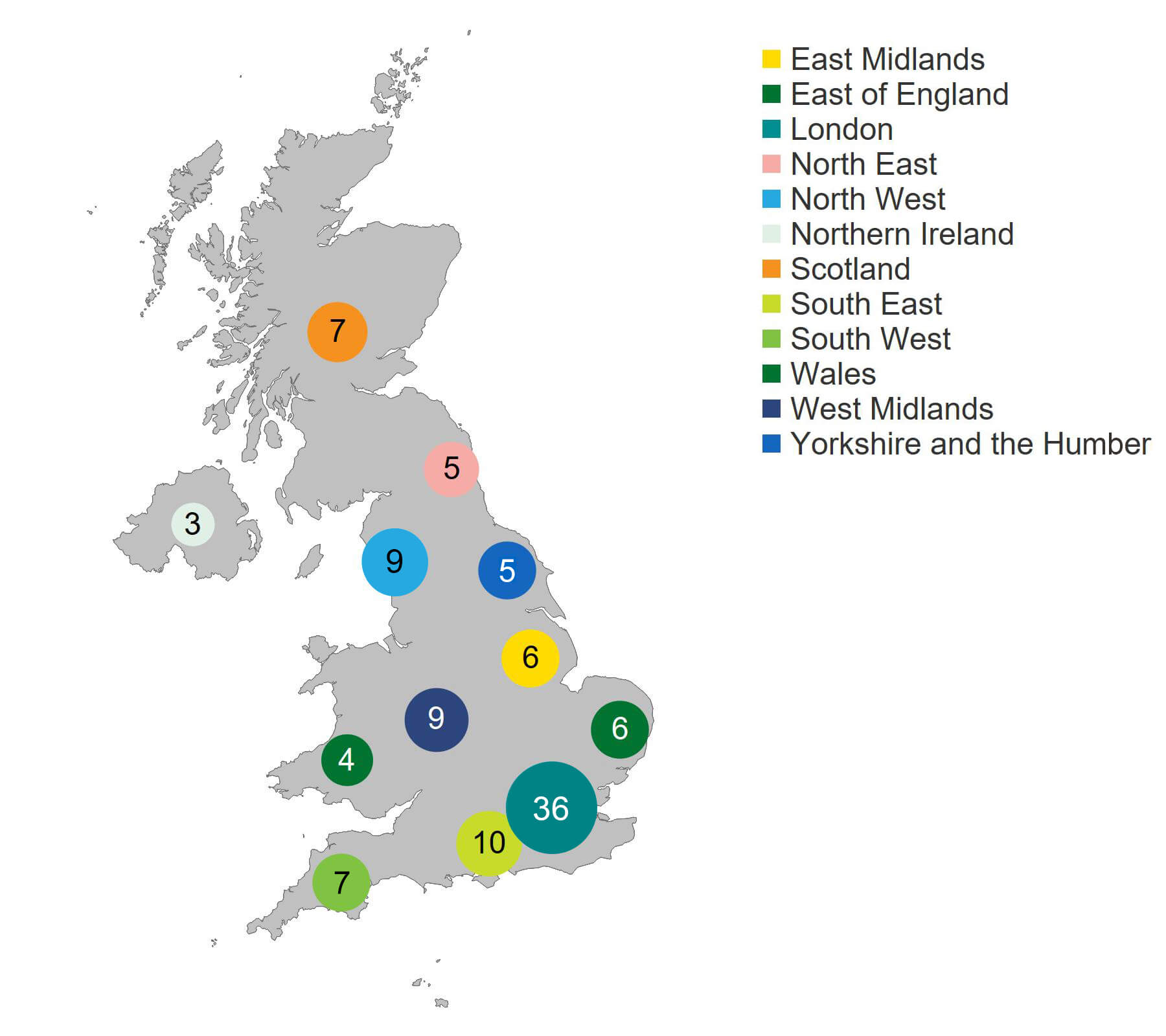
Note figure 4: This figure shows only clinics licensed by the HFEA to perform fertility treatments, meaning that storage only or research clinics are not included.
Download the underlying data for Figure 4 as Excel Worksheet
7. Incidents
Over 99% of the 100,000 cycles of treatment carried out over the last year at HFEA licensed clinics took place without incident.
Incidents in fertility clinics are rare but can be distressing for those patients involved. When incidents do occur, we take them very seriously.
There were 792 incidents reported to the HFEA in 2024/25, 36% more than the 581 incidents reported in 2023/24. Our data shows that 99% of cycles were carried without an incident occurring, or that around eight in every 1,000 cycles at licensed clinics resulted in an incident being reported.
The HFEA will continue to monitor trends over time.
Incidents are graded as A, B or C with A being the most serious. Definitions of grade A, B and C incidents can be found in the Notes on The Fertility Sector 2024/25 towards the end of this document.
The overall increase in incidents reported in 2024/25 was mainly in Grade C incidents. These increased from 289 in 2023/24 to 443 in 2024/25 (+53%). This was due to increases in incidents involving administration (+33%), laboratory process (+105%) and clinical (+88%) incidents.
One reason for the increase over the last year could be that following high-profile incidents in two clinics in 2023/24, clinics are responding to learning shared by the HFEA by more diligently reporting incidents.
The HFEA welcomes the increase in incident reporting. This process allows clinics across the sector to learn from incidents and ensure changes can be made to prevent recurrence. The Quarterly Clinical Governance Updates (shared via our Clinic Focus newsletter) provide clinics with detailed insights into incidents.
There were no Grade A incidents (the most serious) reported in 2024/25, compared with one the previous year, which was the first Grade A incident reported since 2019/20. There were 224 Grade B incidents in 2024/25, 22 more than the previous financial year, but comparable with 2022/23. The total number of Grade B incidents also includes 67 severe/critical OHSS incidents.
Clinic administration incidents usually relate to a breach in confidentiality, for example where an e-mail or letter has been sent to the incorrect recipient. In our National Patient Survey 2024, many patients described situations where clinic administration did not meet their expectations. This ranged from appointments being incorrectly scheduled, receiving incorrect or unclear information about their treatment or billing, to mistakes or errors made by clinic staff. In some cases, these errors resulted in delays in accessing services or cycles being cancelled.
Examples of clinical incidents may include a patient being admitted to hospital and receiving treatment such as IV fluids. Laboratory process incidents can include the loss of an egg during transfer to a dish for IVF.
The number of near misses reported (68 in 2024/25) remain in line with 2023/24. At the time of writing, 57 incidents were yet to be assigned a grade or confirmed to be incidents (referred to as TBA incidents) at the data cut-off date for this report.
On average, clinics reported just under six incidents each in 2024/25, with 42 clinics reporting no incidents. Clinics which carry out more cycles each year may be more likely to experience an incident than smaller clinics, and this should be taken into account when comparing clinics. While some clinics have not reported incidents, this does not automatically indicate better levels of safety, as reporting patterns can vary.
The HFEA issued 14 Field Safety Notices in 2024/25 relating to medical devices used in licensed clinics. The HFEA shares these notices with clinics, even though clinics will already have received them directly from the manufacturer and from the Medicines & Healthcare products Regulatory Agency (MHRA) website. The HFEA does this as a second line of defence ensuring patient safety.
Figure 5. 792 incidents were reported to the HFEA in 2024/25
Number of incidents reported to the HFEA by grade, 2020/21-2024/25
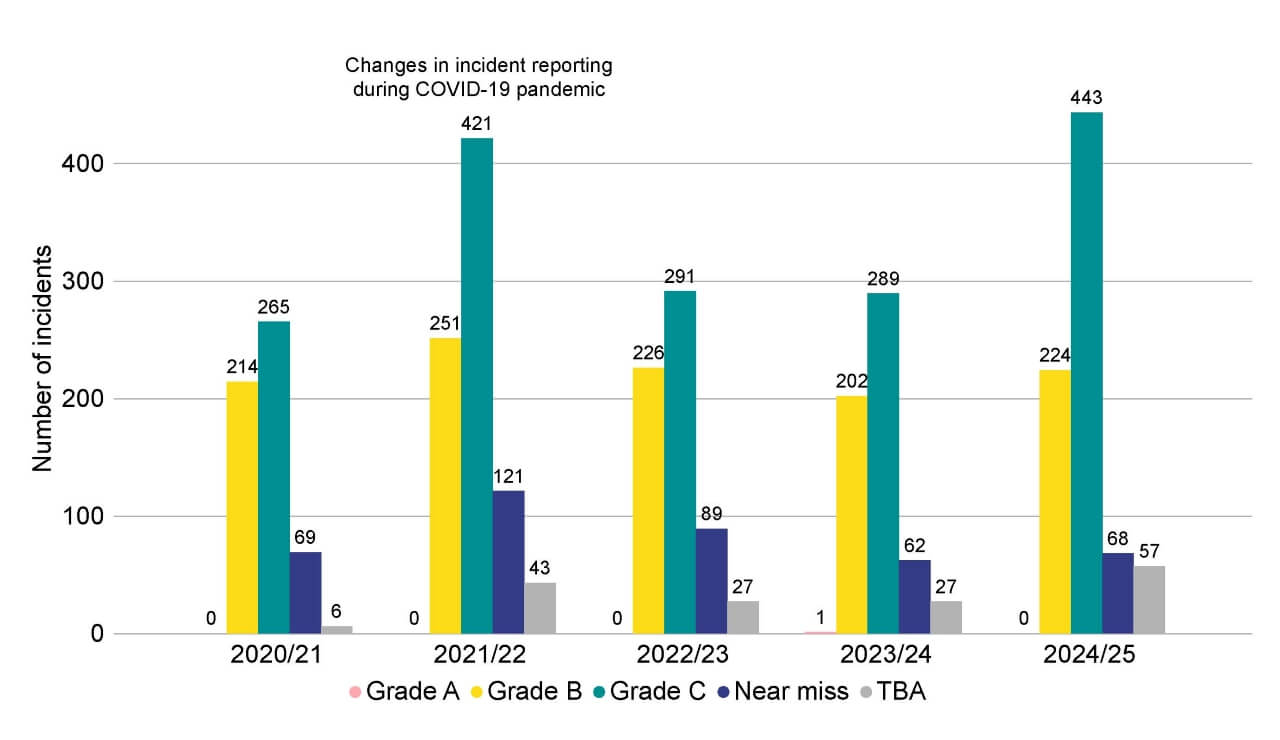
Note figure 5: The 224 Grade B incidents include 67 severe and/or critical OHSS incidents. In previous editions of State of the sector, the total incidents figure included only Grade A, B and C incidents. This total includes Grade A, B and C incidents, as well as Near miss and TBA incidents. TBA incidents are those which were yet to be assigned a grade, or officially confirmed to be an incident, at the data cutoff date of this report. Each financial year shows a snapshot at the time of the respective State of the Sector report, therefore TBA incidents from previous years will have since been assigned a grade but are not detailed in this data.
Download the underlying data for Figure 5 as Excel Worksheet
8. OHSS
Ovarian Hyperstimulation Syndrome (OHSS) is a reaction to fertility drugs taken to increase egg production. OHSS occurs in women who are very sensitive to the fertility medication resulting in too many eggs developing in the ovaries, which become very large and painful. In very rare cases, severe OHSS can be life-threatening. Cases of severe and critical OHSS as a result of fertility treatment must be reported to the HFEA and are included in the total number of incidents.
In 2024/25, there were 67 cases of severe and critical OHSS reported by UK clinics.
None of the cases of severe and critical OHSS reported to the HFEA in 2024/25 related to egg donors.
Figure 6. Severe and critical OHSS incidents in 2024/25 remained consistent with the previous financial years
Number of severe and critical OHSS incidents reported, 2020/21-2024/25
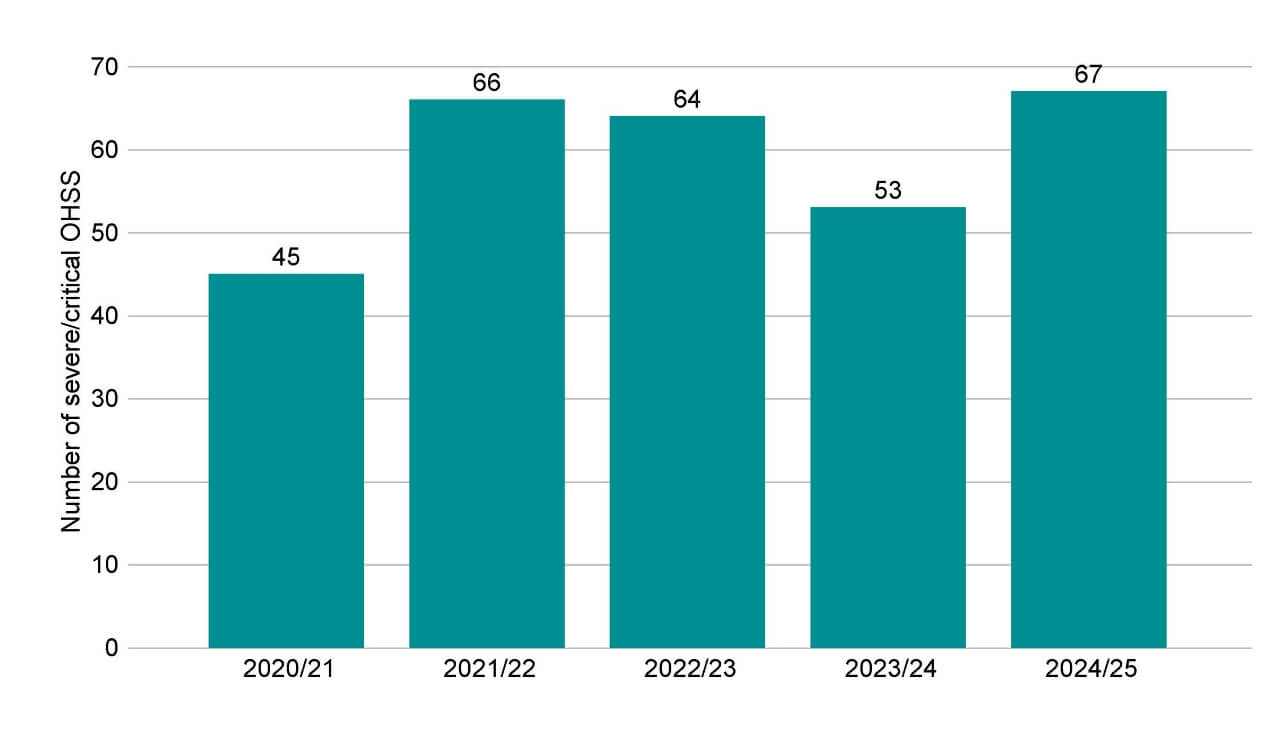
Note figure 6: Treatments in 2020/2021 were impacted by COVID-19 measures introduced by the HFEA. For more information on these, see State of the fertility sector 2020/21. This data includes severe and/or critical OHSS cases only. These severe and critical OHSS incidents are also included in the Grade B incident totals provided in Figure 5. Mild and moderate OHSS cases are not included in this data as these are not classed as incidents.
Download the underlying data for Figure 6 as Excel Worksheet
9. Formal patient complaints
Anyone using HFEA licensed clinics can raise a complaint with us if they are dissatisfied with how a clinic has handled their original complaint. However, unlike some other regulatory bodies, the HFEA does not have a statutory duty to investigate patient complaints. We can only consider a complaint when it indicates a potential breach of the HFE Act 1990 (as amended), licence conditions, or the guidance set out in the Code of Practice. This means we may not be able to review specific medical decisions made during a patient’s care pathway, nor can we intervene to require a clinic to provide a refund or financial compensation.
Patients raise concerns with the HFEA in several ways, including through feedback on clinics’ Choose a Fertility Clinic pages. This section covers formal complaints only.
There were 56 patient complaints received in 2024/25, a decrease of 13 since the previous financial year. This includes 14 formal complaints and 42 informal complaints. Themes include seeking refunds for treatment and being unhappy with their treatment outcome. The HFEA encourages complainants to access the clinic’s complaint process and to have exhausted this process before raising the matter with us.
Complaints are discussed at regular HFEA internal clinical governance meetings to ensure clinics are actively engaging with patient complaints and providing thorough and well-considered responses. We expect clinics to provide a sincere acknowledgement of the complainant’s experience; to explain what, if anything, went wrong; what measures the clinic has put in place to minimise the risk of this happening again, and what the clinic can offer the complainant by way of support. Any feedback provided about clinics that we regulate, however, will be reviewed and will feed into the specific clinic’s inspection process. We would act immediately in the event of serious concerns being raised.
Patient complaint findings are also detailed in our Quarterly Clinical Governance Updates.
Figure 7. Patient complaints in 2024/25 were consistent with the previous financial year
Number of informal and formal complaints received, 2020/21-2024/25
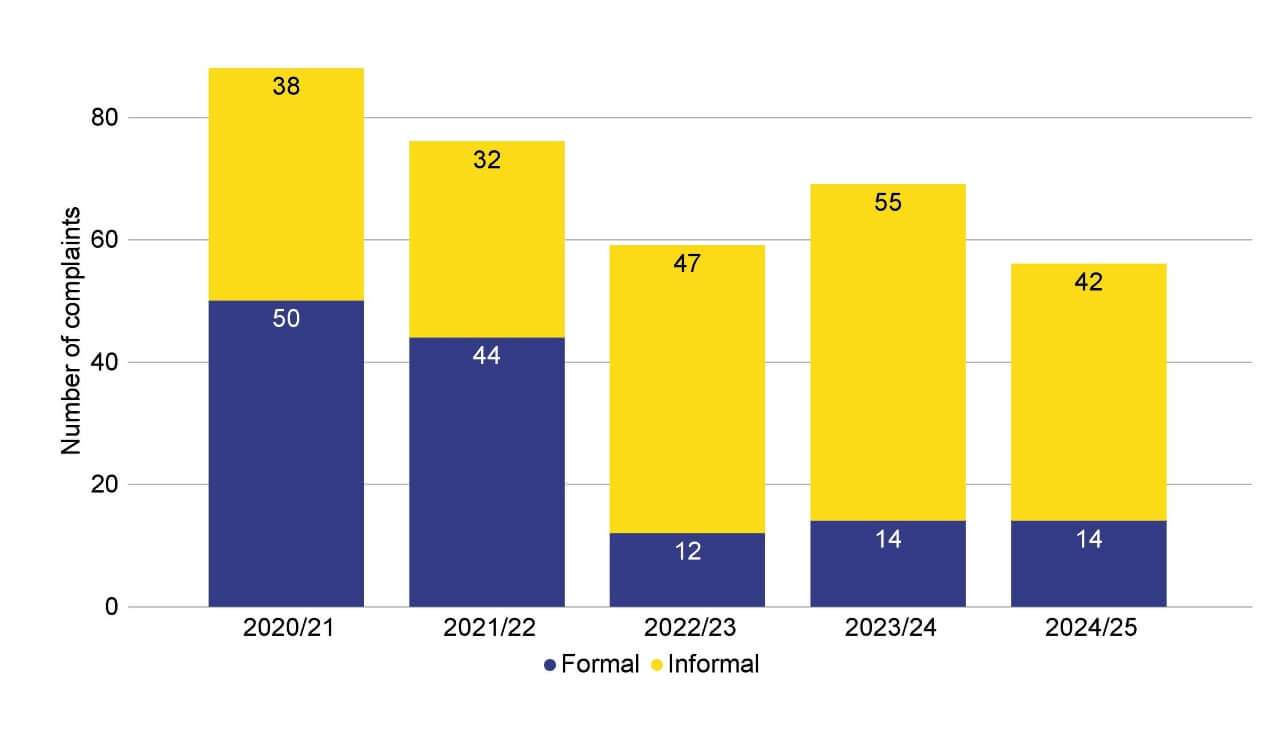
Note figure 7: From 2022/23 the HFEA changed how it handles complaints, leading to fewer being classified as formal complaints.
Download the underlying data for Figure 7 as Excel Worksheet
10. Patient feedback
This year, around 2,500 patients provided feedback via the Choose a Fertility Clinic webpage on clinics licensed by the HFEA. This represents around 5% of patients who received treatment or stored eggs or embryos last year. Clinics are encouraged to ask patients to provide feedback via our website, however, they also have their own feedback mechanisms. Our inspectors review this feedback and, where any concerns are noted, this is raised with the clinic. Patient feedback was positive overall, with most patients (84%) reporting that they would be likely to recommend their clinic to friends or family. Most patients (95%) reported being treated with privacy and dignity, and 92% reported being treated with empathy and understanding by the clinic staff. 93% of patients reported that they understood everything that was happening throughout their treatment.
These findings are in line with the National Patient Survey, which found overall patients were satisfied with their treatment, including how they were treated by clinic staff, and their understanding of their treatment.
11. About our data
This report is compiled from information gathered from our inspections throughout the year and other sources of information, including incident reports, patient feedback, and patient complaints.
This report also uses preliminary treatment data for 2023 from the HFEA Register of fertility treatments. The HFEA has recently launched a new data submission system for licensed clinics and has migrated its fertility treatment and outcomes data to a new database. This data migration has resulted in delays that have prevented the validation of the 2020-2023 treatment and pregnancy data and 2019-2023 birth outcome data. Data validation involves data quality checks that verify treatment, pregnancy and birth outcome data.
The information that we publish is a snapshot of data provided to us by licensed clinics. The figures supplied in this report are from our data warehouse containing Register data as of 1 May 2025. Results are published according to the year in which the cycle was started.
A full list of definitions of classifications of incidents and complaints are available in the underlying dataset. For further information, please see our Quality and methodology report.
About the HFEA
The HFEA is the UK’s independent regulator of fertility treatment and research using human embryos. Set up in 1990 by the Human Fertilisation and Embryology Act, the HFEA is responsible for licensing, monitoring, and inspecting fertility clinics and research centres – and taking enforcement action where necessary – to ensure everyone accessing fertility treatment receives high quality care.
The HFEA is an ‘arm’s length body’ of the Department of Health and Social Care, working independently from Government providing free, clear, and impartial information about fertility treatment, clinics and egg, sperm, and embryo donation.
The HFEA collects and verifies data on all treatments that take place in UK licensed clinics, which can support scientific developments and research and service planning and delivery.
The HFEA is funded by licence fees, IVF treatment fees and a small grant from UK central government. For more information, visit hfea.gov.uk.
Contact us regarding this publication
Media: press.office@hfea.gov.uk
Statistical: intelligenceteam@hfea.gov.uk
Accessibility: comms@hfea.gov.uk
For general information about the HFEA, fertility treatments, and research activity, please visit the HFEA website. For guidance on how to make a complaint about a clinic, please visit our Problems at your fertility clinic webpage.
Notes on The fertility sector 2024/25
- Human Fertilisation and Embryology Authority (HFEA). Fertility Treatment 2023: preliminary trends and figures. 2025
- Inspections are carried out by the HFEA to assess whether clinics comply with essential requirements in providing safe and high-quality care to patients and donors. Inspections are categorised as follows:
- Initial: The first inspection in relation to a new clinic licence application, which covers all areas of regulatory compliance. New licensed clinics usually receive a licence to operate for up to two years for an initial licence, however established clinics and new clinics that are part of a fertility clinic group generally receive a licence for up to four years (five years is the maximum length of a treatment licence permitted by law).
- Interim: An inspection that assesses set themes, as well as any non-compliances identified in previous inspections. Interim inspections include a shorter site visit, are unannounced and are usually carried out at the midpoint of the existing licence.
- Focused interim: An interim inspection focused on particular areas of regulatory compliance. These can be carried out for many reasons, including as a response to a serious incident; to concerns identified in a previous inspection; to a whistleblowing event; or by request of the HFEA licence committee.
- Renewal: An inspection that includes a thorough assessment of all aspects of regulatory compliance, as well as any non-compliances identified in previous inspections. As clinics are usually granted a four-year licence, the renewal inspection is carried out every four years.
- Additional: These inspections are usually focused on a particular area of regulatory compliance, and may be carried out in a number of circumstances including by request of the HFEA licence committee, in the event of a variation of premises, in response to a serious incident and/or whistleblowing, or in the event of any other concerns identified.
- Non-compliances are graded as:
- Critical: An area of practice that poses a significant risk of causing harm to a patient, donor, embryo or to a child who may be born as a result of treatment services; or a significant shortcoming from the statutory requirements.
- Major: An area of practice that poses an indirect risk to the safety of a patient, donor, embryo or to a child born as a result of treatment services. This area of non-compliance may also indicate a major shortcoming from the statutory requirements and/or indicate a failure by the Person Responsible to carry out their legal duties.
- Other: An ‘other’ area of practice that requires improvement is any area of practice, which cannot be classified as either a critical or major area of non-compliance, but which indicates a departure from statutory requirements or good practice.
- In 2023 there were approximately 99,000 cycles of treatment and storage involving approximately 63,000 patients:
- Approximately 83,000 treatment cycles (around 77,500 IVF; 5,500 DI)
- Approximately 16,000 storage cycles (around 6,900 egg storage cycles; 9,000 embryo storage cycles)
- With an additional approximate 1,400 new egg donor registrations, and 1,000 new sperm donor registrations.
- Incidents are graded as:
- Grade A: involves severe harm to one person, or major harm to many
- Grade B: involves serious harm to one person, or moderate harm to many
- Grade C: involves minor harm
- Near miss: an event not causing harm, but with the potential to cause injury or ill health.
- Complaints are classified as:
- Formal: where the patient/donor has received a response from the clinic but remains dissatisfied, and/or where the clinic has entered an extensive dialogue with the complainant and at the end of this process feel they have done all they can to resolve the complaint, therefore advising the complainant to contact us. Complaints relating to an incident or complaints that relate to complex issues requiring further input from us are also classified as formal complaints.
- Informal: complaints that have not been raised with the clinic or complaints still going through the clinic’s complaint process.
| Publication date: |
|---|

